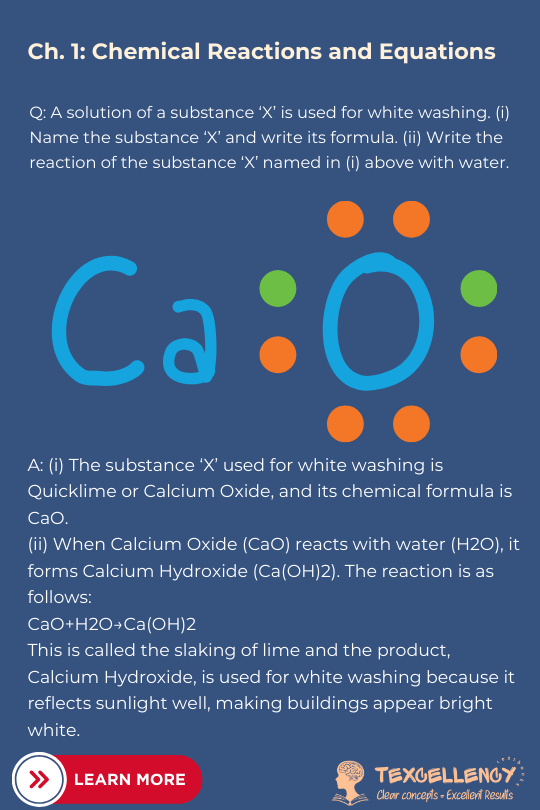
A: (i) The substance ‘X’ used for white washing is Quicklime or Calcium Oxide, and its chemical formula is CaO. (ii) When Calcium Oxide (CaO) reacts with water (H2O), it forms Calcium Hydroxide (Ca(OH)2). The reaction is as follows: CaO+H2O→Ca(OH)2 This is called the slaking of lime and the product, Calcium Hydroxide, is used for white washing because it reflects sunlight well, making buildings appear bright white.
This question belongs to the first chapter of the CBSE 10th Std Science book: Chemical Reactions and Equations